Useful for Observation of Cell Physiological Functions Over Time! Organelle Imaging Reagents for Living Cells
Date:September 30 2024Web Page No:520072

Funakoshi Co.,Ltd.
Live-cell imaging has garnered attention as an excellent method for observing cellular physiological phenomena over time, with various imaging reagents being utilized for different organelles. Since it allows for the observation of cellular behavior in living cells, it is expected to be a useful technique for monitoring dynamic changes in physiological phenomena caused by extracellular stimuli or changes in gene expression. However, there is a possibility that the imaging reagents themselves may affect the cells, so there is a need for reagents that are low in toxicity and do not interfere with physiological functions.
We offer a unique range of "MADE IN JAPAN" organelle imaging reagents specifically designed for live cells. Each of these reagents has distinct advantages over existing alternatives, so please refer to them for more details.

- ☞ For low-toxicity and genome dynamics observation of the nucleus!
- ☞ For long-term observation with medium-change for endoplasmic reticulum!
- ☞ For observing cytoplasmic morphology with washable reagent!
- ☞ For accurate observation of cell membrane positioning!
- ☞ For detecting tiny lipid droplets in non-adipocytes!
- ☞ For selectively visualization of Golgi apparatus!
Product Overview
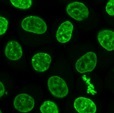
NucleoSeeing™
NucleoSeeing™ is a live-cell imaging nuclear staining reagent that specifically binds to DNA and emits green fluorescence. It demonstrates a high signal-to-noise (S/N) ratio not only in animal cells and tissues but also in leaf cells of Arabidopsis thaliana. It is excellent for observing nuclear dynamics in live cells. Additionally, it can be applied for nuclear pH sensing.
Long time-lapse imaging
HeLa cells were seeded onto a glass-bottom dish and treated with 0.5 μM NucleoSeeing™ in DMEM (+10% FBS) for 1.5 hours after 20 hours. Time-lapse imaging was performed using a confocal laser microscope under unwashed conditions at 10-minute intervals for a total of 20 hours (excitation/fluorescence wavelengths: 488 nm/500–600 nm, 60x oil lens). The process of mitotic chromosome division was observed, and the cell proliferation process could be monitored.
※ Note: When performing long-term time-lapse imaging with this reagent, it is recommended to observe the cells without changing the culture medium, keeping the reagent included in the medium.
For details, please see ☞ NucleoSeeing™(Live Nucleus Green).
ERseeing™
ERseeing™ is an irreversible ER staining reagent for live-cell imaging. Compared to conventional glibenclamide-fluorophore conjugated ER dyes, it has a smaller pharmacological effect on cell function and, being irreversible, it allows observation even after medium exchange. Excitation/fluorescence wavelengths: 509 nm/524 nm (compatible with typical green dye observation conditions such as FITC)
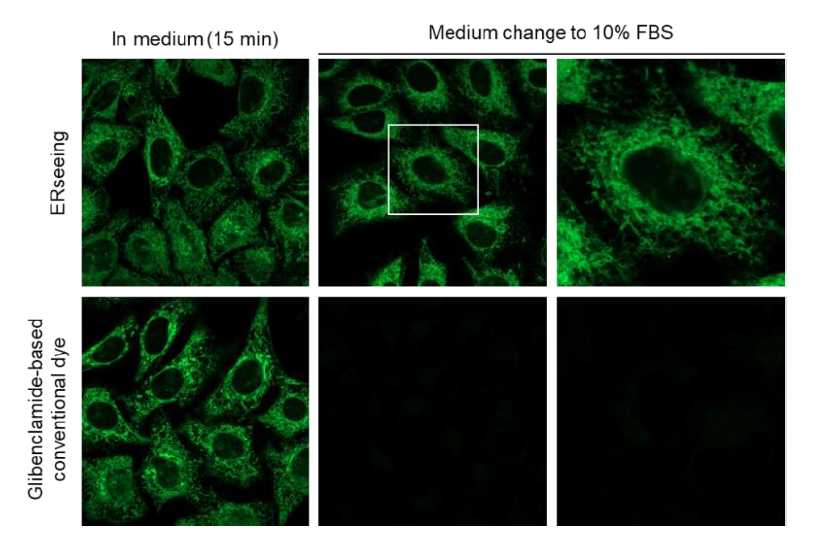
Evaluation of intracellular retentivity
HeLa cells were treated with ERseeing™ and the conventional glibenclamide-based dye in serum-free medium and stained for 15 minutes before observation (left). The medium was then replaced with 10% FBS-containing medium and observed again. After washing, the fluorescent signal of the glibenclamide-based dye significantly disappeared, while ERseeing™, being irreversible, maintained a strong signal even after washing. By using ERseeing™, it is possible to culture cells in FBS-containing medium after ER staining, enabling various imaging applications.
For details, please see ☞ ERseeing™(Endoplasmic Reticulum Green).
CytoSeeing™
CytoSeeing™ is a novel cytoplasmic staining reagent that rapidly stains the cytoplasm of live cells simply by adding it to the culture medium. After observing the cytoplasm under fluorescence, the fluorescence can be easily removed from the cells by exchanging the medium. It is useful for temporary cellular morphology observations.
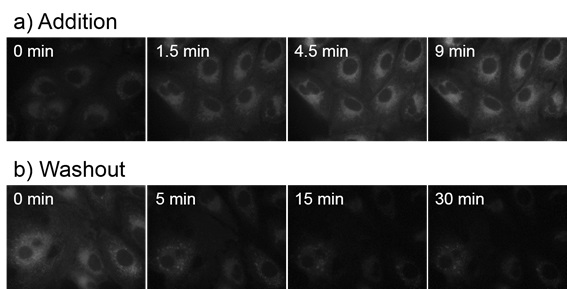
Time-dependent incorporation and washout of CytoSeeing™ in A549 cells
- a) CytoSeeing™ (10 μM) was added and incubated. CytoSeeing™ was completely incorporated into cell by 9 minutes.
- b) CytoSeeing™ incorporated cells were washed and incubated in fresh medium (without CytoSeeing™). Almost CytoSeeing™ was removed in 30 minutes.
For details, please see ☞ CytoSeeing™(Reversible Cytoplasm Blue).
Ap3
Ap3 is the first non-fluorescent SHG (Second Harmonic Generation) dye that does not emit fluorescence, which could interfere with SHG signal imaging. Unlike fluorescent membrane dyes, only the signal from the cell membrane can be observed, enabling precise analysis of membrane positioning. Additionally, the signal intensity correlates with membrane potential, making it useful for membrane potential imaging.
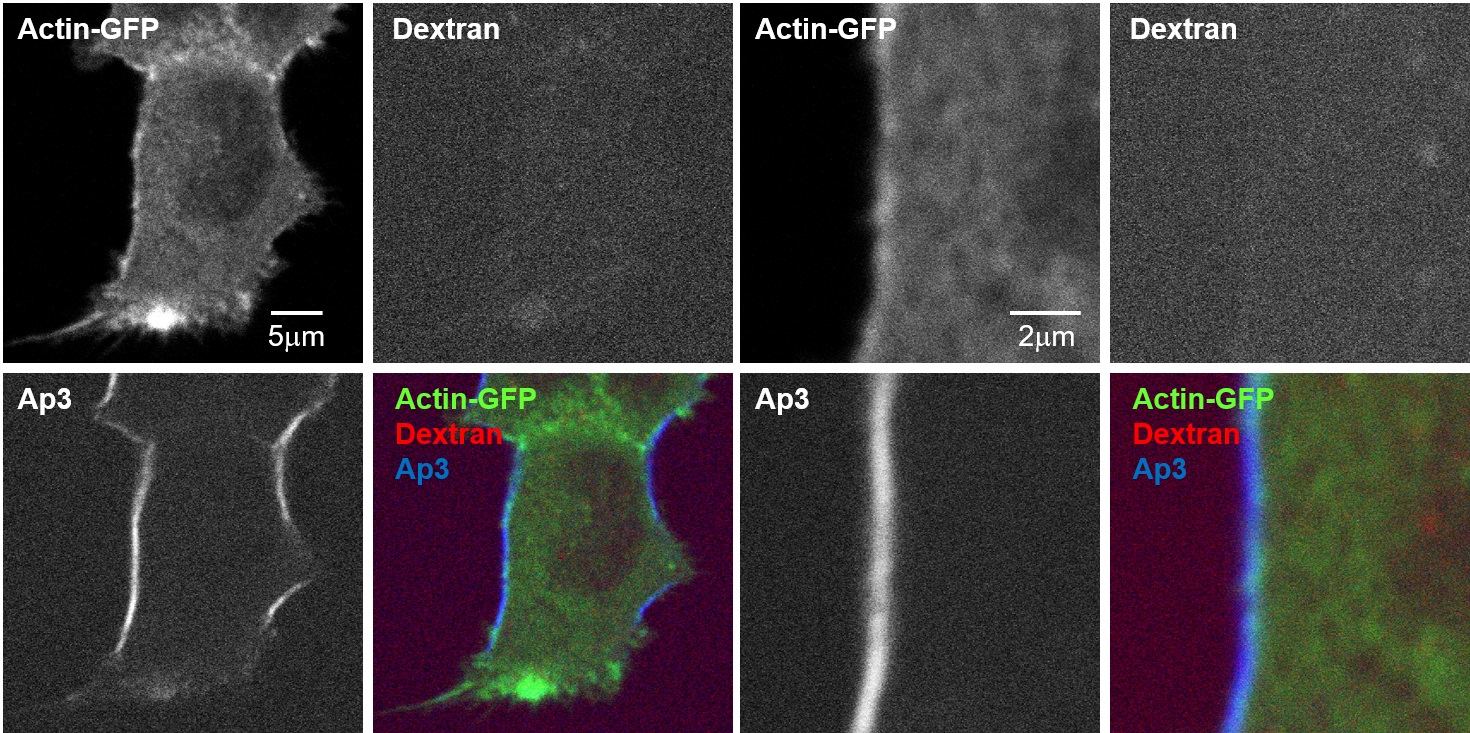
Analysis of vesicle dynamics and tubulin beneath the plasma membrane using multimodal two-photon microscopy
In cells stained with Tubulin Tracker for microtubules, vesicles after endocytosis were stained with 10kDa-Dextran Alexa 594, and the plasma membrane was stained with Ap3. These components were simultaneously visualized using a multimodal two-photon microscope.
Accurate identification of plasma membrane's position by Ap3-SHG, which is plasma membrane-specific and fluorescence signal-separable dye, allows for precise measurement of distances from the plasma membrane to microtubules and to vesicles moving just beneath the plasma membrane.
Reference
- Nuriya, M., et al., "Multimodal two-photon imaging using a second harmonic generation-specific dye", Nat. Commun.,
7,11557 (2016).( )
) - Mizuguchi T., et al, "High-Resolution Plasma Membrane-Selective Imaging by Second Harmonic Generation", iScience,
9, 359~366 (2018).( )
)
For details, please see ☞ Ap3 (SHG Imaging Dye).
LipiDye™Ⅱ
This is a highly sensitive lipid droplet staining reagent that excels in long-term live-cell imaging. In addition to its high specificity for lipid droplets, it boasts low toxicity and extremely high photostability. It is useful for long-term observations over several days, live-cell imaging of lipid droplet fusion and degradation processes, and the visualization of ultrafine lipid droplets using super-resolution microscopy.
Reference
- Taki, M., et al., ACS Mater. Lett., in press
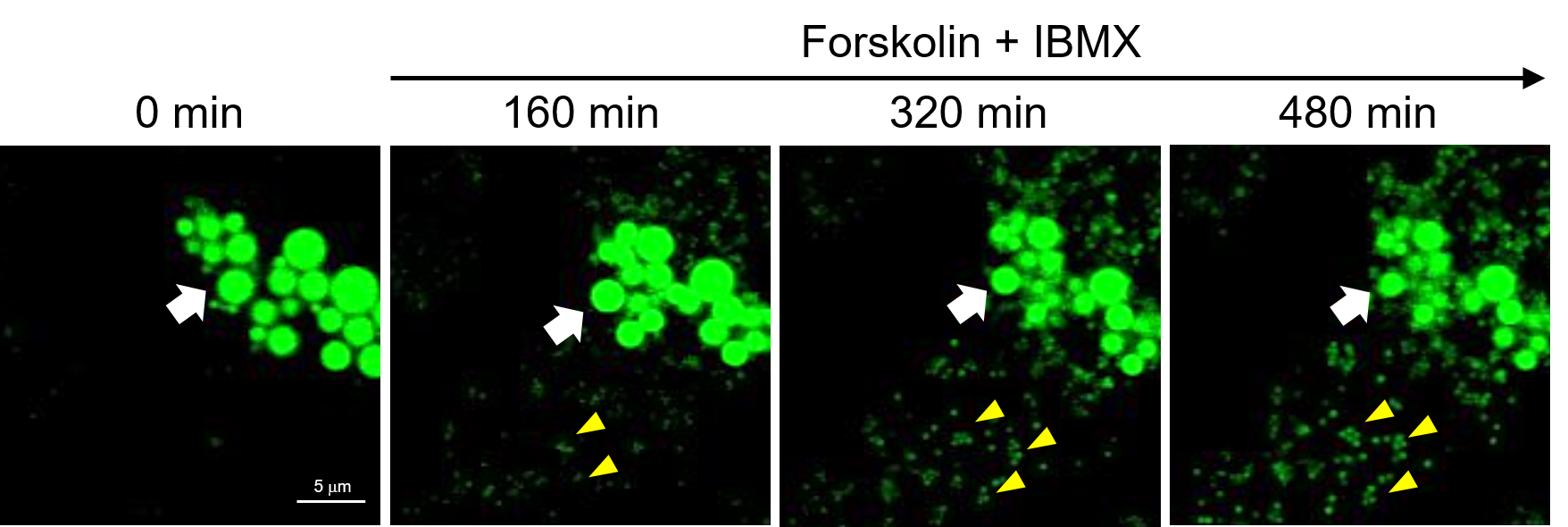
Time-lapse observation of lipid droplet decomposition and neogenesis
Differentiated 3T3-L1 cells were treated with Forskolin (10 μM), an adenylate cyclase activator, and IBMX (100 nM), a phosphodiesterase inhibitor, to induce the breakdown of triacylglycerol, and the reduction of lipid droplets was observed via time-lapse imaging (Z-stack imaging) using LipiDye™Ⅱ (confocal laser microscope: excitation 473 nm/fluorescence 490–540 nm, 800 minutes, imaging every 4 minutes, 15 Z-stack images per session). It was confirmed that the originally large lipid droplets (white arrow: ⇨) gradually shrank over time. Additionally, many small lipid droplets (yellow arrow: ◄) were generated in a time-dependent manner. LipiDyeⅡ, with its extremely high photostability, showed no fading concerns and a high signal-to-noise (S/N) ratio, allowing even the observation of minute newly formed lipid droplets. This demonstrates the reagent's ability to quantitatively monitor the increase and decrease of lipid droplets.
For details, please see ☞ LipiDye™Ⅱ.
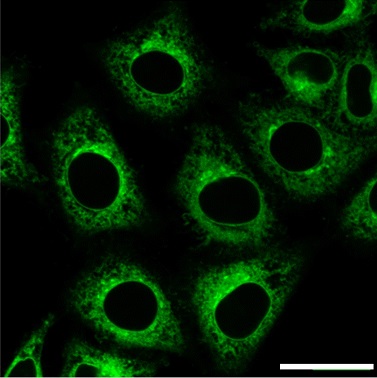
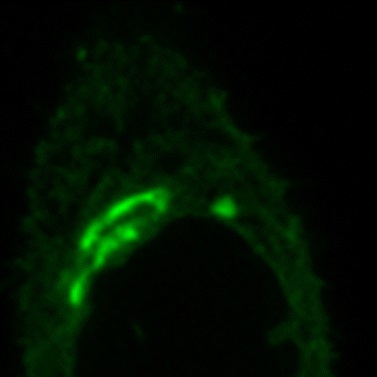
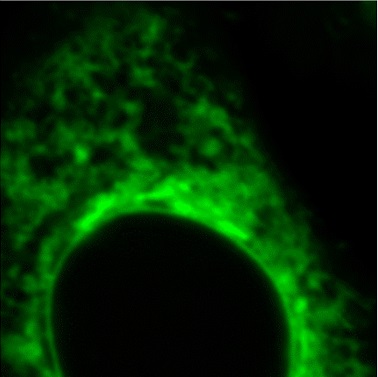
GolgiSeeing™
GolgiSeeing™ is a novel and innovative fluorescent dye for selective Golgi apparatus staining. Compared to conventional ceramide-based Golgi staining reagents, GolgiSeeing™ does not require complicated procedures and suppresses non-specific localization to the endoplasmic reticulum.
| GolgiSeeing™ | Ceramide-FL | |||
| Just addition | Just addition | Step-wise protocol | ||
 |
 |
 |
||
 |
 |
 |
||
Comparison between GolgiSeeing™ & ceramide-based reagent
HeLa cells were treated with GolgiSeeing™ or ceramide-FL (as BSA complex). In the case of GolgiSeeing™, the protocol is a simple addition of GolgiSeeing™ into media and incubated. After washing cells with BSA containing media, fluorescent images were captured by confocal laser microscopy (Ex 488 nm/Em 500-600 nm). On the other hand, ceramide-FL (BSA complex) -staining was performed by two protocols, simple addition or stepwise temperature-controlled protocol. This stepwise protocol requires over 1 hour. The ceramide-FL probe stains not only the Golgi apparatus but also ER structure with high intensity non-specifically. GolgiSeeing™ was able to visualize Golgi apparatus selectively and suppressed non-specific ER staining.
For details, please see ☞ GolgiSeeing™(Golgi Apparatus Green).
Product Lineup
| Organelle | Name (Code) |
Example | Lieve cell | Fixation after staining | Fluorescence | Features & Advantages |
|---|---|---|---|---|---|---|
| Nucleus | NucleoSeeing™ (#FDV-0029) |
 |
Good | Good | Green Ex: 488 nm Em: 520 nm |
|
| Endoplasmic reticulum (ER) | ERseeing™ (#FDV-0038) |
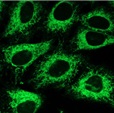 |
Good | Good | Green Ex: 509 nm Em: 524 nm |
|
| Cytoplasm & Internal cell membrane |
CytoSeeing™ (#FDV-0017) |
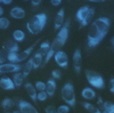 |
Good | Bad | Blue Ex: 345 nm Em: 456 nm |
|
| Cell membrane | Ap3 (#FDV-0008) |
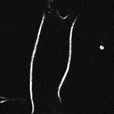 |
Good | Bad | SHG Ex: 950 nm Em: < 470 nm |
※ Two-photon excitation microscopy system and special filter for SHG imaging is required. Please see the product page for details. |
| Lipid droplet | LipiDye™Ⅱ (#FDV-0027) |
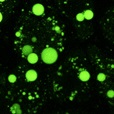 |
Good | Good | Green Ex: 400-500 nm Em: 490-540 nm |
|
| Golgi apparatus | GolgiSeeing™ (#FDV-0053) |
 |
Good | Bad | Green Ex: 480 nm Em: 520 nm |
|
CONTACT
export@funakoshi.co.jp
- ※Prices on our website are for your reference only. Please inquire your distributor for your prices.
- ※Please note that Product Information or Price may change without notice.