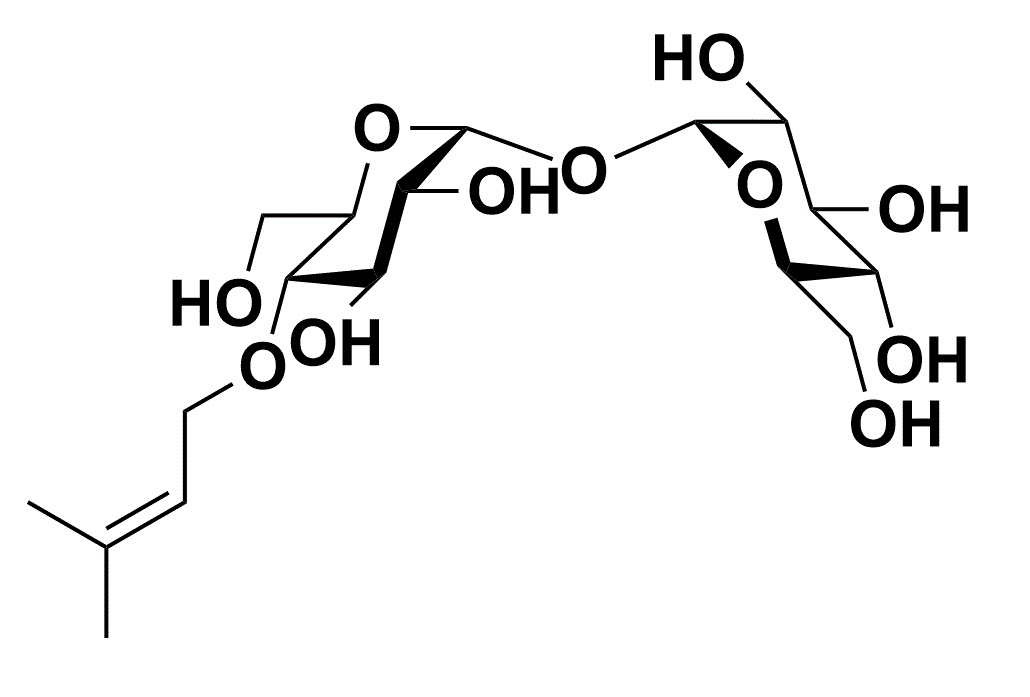
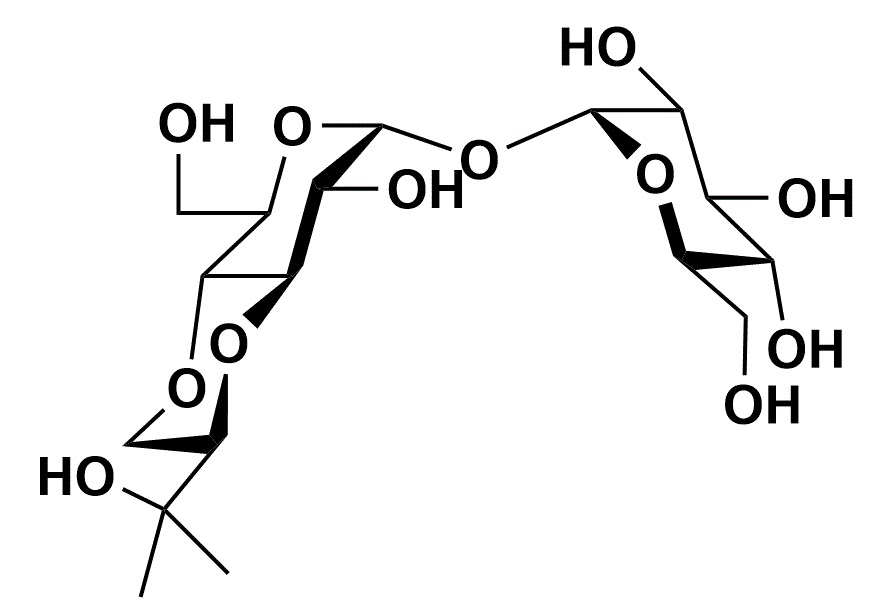
Biologically stable Trehalose analogue suitable for chemical modification 4-Trehalosamine
Date:June 24 2025Web Page No:520088
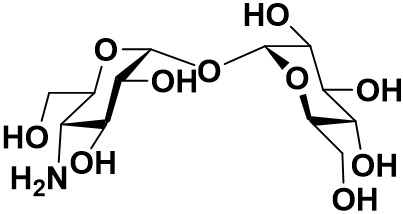
4-Trehalosamine is a trehalose analogue which is expected to have similar functions of it and was known as biologically stable against digestion by mammalian trehalase.
* A derivative of 4-trehalosamine, IMCT4-C14, which is a sugar surfactant and lentztreharose which is an analogue of trehalose are also available. Click here!
* This product is for research use only.
- Comparison of trehalose and 4-trehalosamine
- Specification
- Application Note
- Reference
- Product Information
- Related Products: IMCTA-C14 / Lentztrehalose
Comparison of trehalose and 4-trehalosamine
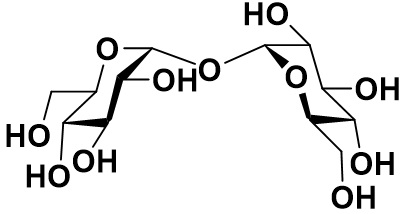
Trehalose is known as a disaccharide which is a glucose dimer. A lot of organisms in nature produce, store, and use trehalose to obtain sugar storage and desiccation tolerance. Mammalians including human have trehalose a specific glycosidase and utilize the enzyme to obtain energy from the sugar in food. It is also noticed the compound has the medication effectiveness. As an example of therapeutic efficacy of it against neurodegenerative diseases2), it was reported that this compound extended lifespan in a transgenic mouse model of Huntington disease.4) Although it was reported some studies to use trehalose as therapeutic or preventive medicines against fatty liver, arteriosclerosis, stroke, and diabetes, it is also known that it is unstable due to easy degradation by microorganisms.5)
Although 4-trehalosamine which is substituted one hydroxyl group to the amino group in trehalose is expected to have similar efficacy of trehalose, it is high stable and cannot be hydrolyzed by trehalases in mammalian.5) In oral administration to mice, it was reported that most of 4-trehalosamine was excreted without degradation, modification, nor increase of blood glucose level after a part of it was circulated in blood. In bacterial culture, the amount of 4-trehalosamine was decreasing because it might be caused by modification of the amino group in most cases. As it did not increase the growth rate of bacteria in media with low carbon source, however, it was confirmed that 4-trehalosamine should not be hydrolyzed under the condition. In addition, it was observed that 4-trehalosamine had protective effect from desiccation and inhibitory activity of starch retrogradation, which were similar or slightly stronger than trehalose.5) Furthermore, its functions which trehalose does not have is pH buffering action at neutral pH stronger than Tris which is a well-known buffer solution.5) Therefore, 4-trehalosamine might be expected to be more suitable for versatile applications than trehalose because of a moisturizing protectant with pH buffering action.
As production volume of 4-trehalosamine is relatively high these days, it is expected to be close to the commercialization stage.
Trehalose derivatives are also synthesized from 4-trehalosamine.
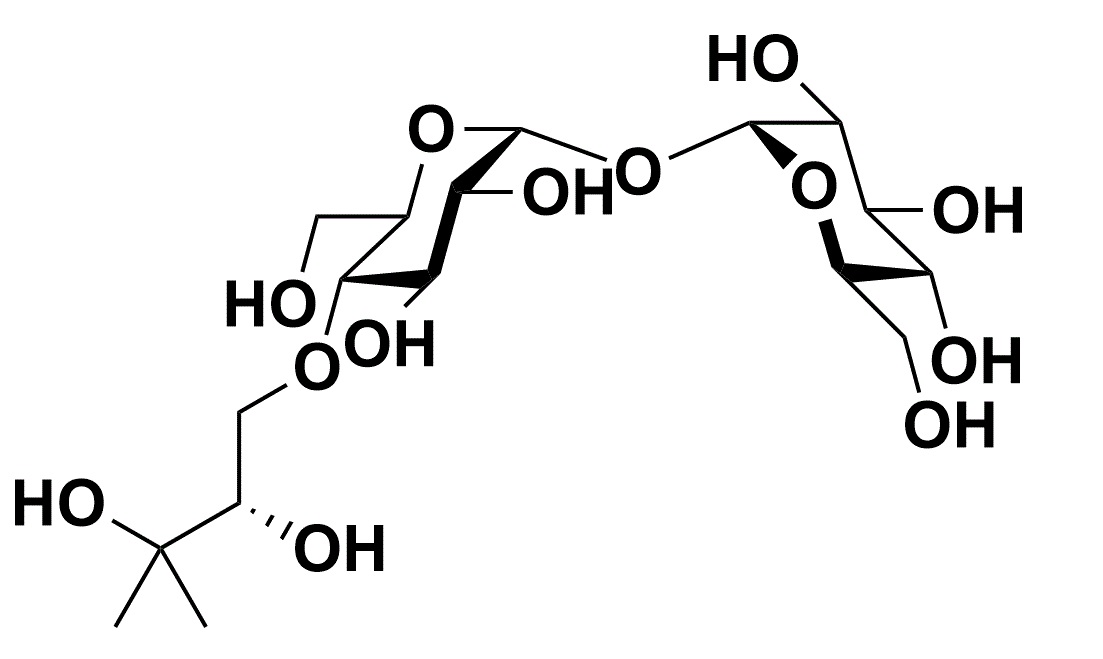
One example of it is IMCTA-C14 which is a N-tetradecyl derivative and functions as a sugar surfactant.
Specification
| CAS# | 51855-99-3 |
|---|---|
| Molecular Formula | C12H23NO10 |
| Molecular Weight | 341.313 |
| Purity | >90% (qNMR) |
| Solubility | Soluble in H2O, DMSO, and MeOH |
Application Note
- Unlike trehalose, 4-trehalosamine was not hydrolyzed by incubation with a mammalian trehalase for 25 hours.5)
- In contrast to trehalose, oral administration of 4-trehalosamine to mice did not cause increasing of blood glucose level and excreted an original substance.5)
- 4-Trehalosamine showed strong pH buffering action at around pH 7.0 similar to that of Tris buffer.5)
- 4-Trehalosamine showed stronger inhibitory activity of starch retrogradation than that of trehalose.5)
- 4-Trehalosamine showed protective effects from desiccation of protein and lyophilization of microorganisms stronger than those of trehalose.5)
Reference
- Naganawa, H., et al., J. Antibiot., 27 (2), 145~146 (1974). [PMID:4826088]
- Khalifeh, M., et al., Bioessays, 42 (8), e1900195 (2020). [PMID:32519387]
- Khalifeh, M., et al., Neural. Regen. Res, 16 (10), 2026~2027 (2021). [PMID:33642389]
- Tanaka, M., et al., Nat. Med., 10 (2), 148~154 (2004). [PMID:14730359]
- Wada, S., et al., Adv. Bio., 6 (6), e2101309 (2022). [PMID:35297567]

Product Information
[Date : March 03 2026 00:09]
| Detail | Product Name | Product Code | Supplier | Size | Price | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
4-Trehalosamine, Trehalase Resistant, Preventive Effect on Starch Retrogradation DatasheetThis may not be the latest data sheet. |
15612 | IMCInstitute of Microbial Chemistry | 25 mg | $500 | |||||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||||||||
[Date : March 03 2026 00:09]
4-Trehalosamine, Trehalase Resistant, Preventive Effect on Starch Retrogradation
DatasheetThis may not be the latest data sheet.
- Product Code: 15612
- Supplier: IMC
- Size: 25mg
- Price: $500
| Description |
M.W.: 341.313 Purity : >90% (qNMR) Molecular Formula : C12H23NO10 Solubility : Soluble in MeOH, H2O, and DMSO 4-Trehalosamine is a trehalose analogue having trehalase tolerance. This analogue showed strong buffering action at neutral pH similar to that of Tris buffer. In addition, it was reported that this compound had protective effect from desiccation and inhibitory activity of starch retrogradation, which were similar or slightly stronger than trehalose. |
||
|---|---|---|---|
| Storage | -20°C | CAS | 51855-99-3 |
| Link |
|
||
Related Products: IMCTA-C14 / Lentztrehalose
IMCTA-C14 (Trifluoroacetate)
IMCTA-C14 is a N-tetradecyl derivative of 4-trehalosamine and functions similarly to a sugar surfactant with a complex of a sugar as a hydrophilic group and long chain aliphatic hydrocarbon. This compound is stable against trehalases in mammalian and was not observed hydrolysis by porcine one. In addition, it showed high efficiency of protein extraction and solubilization without denaturing proteins.
* See details of IMCTA-C14, click here!
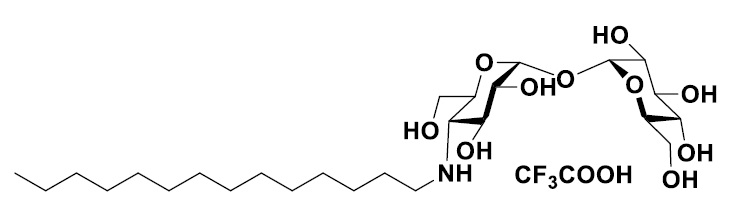
Structure of IMCTA-C14 (Trifluoroacetate) (#15613)
Lentztrehalose
Lentztrehalose A, B, and C are trehalose analogues isolated from the soilid-state culture of Lentzea sp. ML457-mF8. Unlike trehalose, these lentztrehalose analogues are stable to porcine kidney trehalase and not broken down by various microorganisms that metabolize trehalose.
* See details of Lentztrehalose, click here!
CONTACT
export@funakoshi.co.jp
- ※Prices on our website are for your reference only. Please inquire your distributor for your prices.
- ※Please note that Product Information or Price may change without notice.