Glyoxalase I (GloΙ) Inhibitor COTC
Date:July 07 2021Web Page No:95016
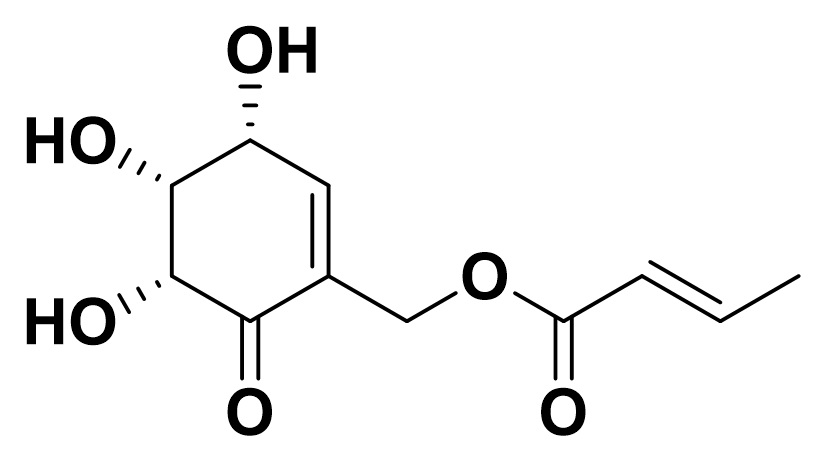
2-Crotonyloxymethyl-(4R,5R,6R)-4,5,6-trihydroxycyclohex-2-enone (COTC) is an inhibitor of glyoxalase I isolated from the culture of Streptomyces griseosporeus MD287-CF31, 2).
COTC shows a strong growth inhibition of HeLa cells and Ehrlich ascites carcinoma with low toxicity (LD50 mice:90 mg/kg i.v.)1). COTC reacts with glutathione or other SH-compounds to form corresponding thioethers2, 4). Synthesis of COTC derivatives for antitumor activity has also been reported6).
6-epi-COTC, one of the diastereomers of COTC and COMC, a synthetic analog of COTC with a simplified structure are also available.
Glyoxalase and dicarbonyl stress
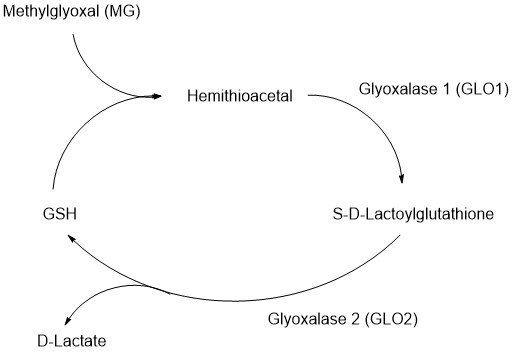
Glyoxalase system is known as an enzymatic network having an important role of detoxication in a lot of organisms. This enzymatic network is composed of glyoxalase 1 (GLO1), glyoxalase 2 (GLO2), and reduced form of Glutathione (GSH), and detoxifies harmful metabolites such as methylglyoxal (MG) in cells.7)
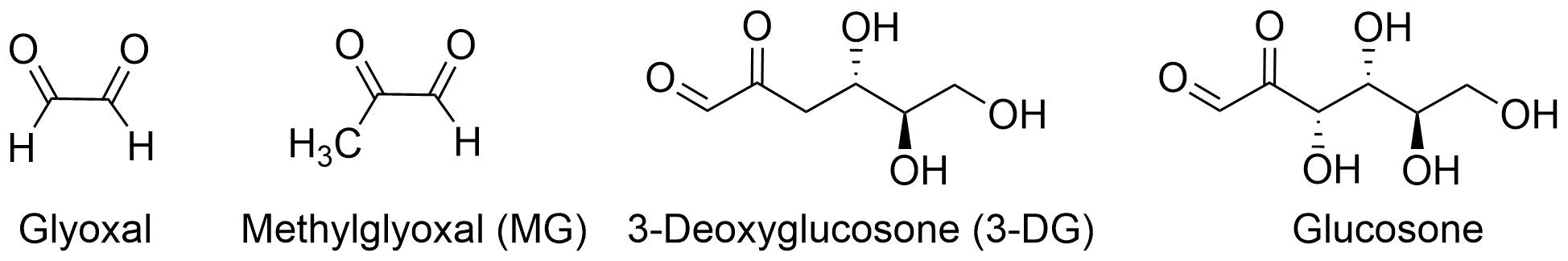
MG has two sets of carbonyl groups (C=O), and is also called a dicarbonyl compound.
It is known that the dicarbonyl compounds as metabolites from the system are composed from alpha-oxoaldehydes glyoxal, 3-deoxyglucosone (3-DG), and glucosone etc.
These dicarbonyl compounds has high reactivity to DNA and protein to generate dicarbonyl stress which cause functional disorders of cells and tissues in disease and senescence. Then, the undesired metabolites cause adversely affect to physiological system. CLO1 in cytosol suppresses generation of the harmful metabolites by degradation of dicarbonyl compounds such as MG. First, GLO1 metabolizes MG to S-D-lactoylgluthathione. Then, GLO2 catalyzes hydrolysis of S-D-lactoylgluthathione into D-lactate, and regenerate GSH which was consumed by the metabolic reaction of GLO1.8)
Dicarbonyl compounds to cause dicarbonyl stress, especially MG and advanced glycation end products (AGE) produced from MG which is a precursor of it, relate variety of diseases including diabetes, cardiovascular diseases, neurodegenerative diseases, cancer disease etc. It can be said that GLO1 is an important rate-determining enzyme (key enzyme) that protects glycation in vivo. Therefore, GLO1 is expected to an important drug target, and to determine its regulatory mechanism is of interest to researchers.7)
Specification
| CAS# | 57449-30-6 |
|---|---|
| Molecular Formula | C11H14O6 |
| Molecular Weight | 242.227 |
| Source | Streptomyces griseosporeus MD287-CF3 |
| Purity | >98% (HPLC) |
| Solubility | Soluble in MeOH, DMSO and H2O Insoluble in n-Hexane |
Application Note
- OCTC shows IC50 values against rat liver crude glyoxalase and yeast glyoxalase I at 1.8 mM and 1.4 mM, respectively, in a reaction mixture containing 1.59 mM of reduced glutathione and preincubated for 3 minutes before enzyme addition.1, 3)
- COTC blocks enzymatic activity of alkaline phosphodiesterase derived from murine lymphoblastoma L5178Y cells (IC50: 60 μg/ml). COTC and aclarubicin exhibits synergistic activity on aclarubicin-resistant cells, but not on the parental cells.5)
Reference
- Takeuchi T., et al., J. Antibiot., 28 (10), 737~742 (1975). [PMID:1102510]
- Chimura H., et al., J. Antibiot., 28 (10), 743~748 (1975). [PMID:1184466]
- Matsuda A., et al., Japanese Patent, JPS52113946A (1977).
- Huntley C.F., et al., Org. Lett., 2 (20), 3,143~3,144 (2000). [PMID:11009366]
- Sugimoto Y., et al., J. Antibiot., 35 (9), 1,222~1,230 (1982). [PMID:7142023]
- Tony K.M.S., et al., Bioorg. Med. Chem. Lett., 22 (24), 7,562~7,565 (2012). [PMID:23102892]
- He, Y., et al., Biomed. Pharmacother., 131, 110663 (2020). [PMID:32858501]
- Rabbani, N., et al., Oxidative Stress: Eustress and Distress, Chapter 36, p.759~777, (2020), Edited by Sies, H., Elsevier

[Date : February 23 2026 00:07]
| Detail | Product Name | Product Code | Supplier | Size | Price | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
COTC, Glyoxalase-I Inhibitor DatasheetThis may not be the latest data sheet. |
14675 | IMCInstitute of Microbial Chemistry | 1 mg | $500 | |||||||||||||||||||||||||||||||

|
|
||||||||||||||||||||||||||||||||||
[Date : February 23 2026 00:07]
COTC, Glyoxalase-I Inhibitor
DatasheetThis may not be the latest data sheet.
- Product Code: 14675
- Supplier: IMC
- Size: 1mg
- Price: $500
| Description |
M.W.: 242.227 Purity : >90% (HPLC) Molecular Formula : C11H14O6 Solubility : Soluble in MeOH, DMSO, and H2O, Insoluble in hexane, blocks enzymatic activity of alkaline phosphodiesterase derived from murine lymphoblastoma L5178Y cells (IC50: 60ug/ml), glyoxylase I in rat liver crude (IC50: 1.8 mM), and in yeast (IC50: 1.4 mM) |
||
|---|---|---|---|
| Storage | -20°C | CAS | 57449-30-6 |
| Link | |||
CONTACT
export@funakoshi.co.jp
- ※Prices on our website are for your reference only. Please inquire your distributor for your prices.
- ※Please note that Product Information or Price may change without notice.