NADH:Ubiquinone oxidoreductase (Complex I) inhibitor Piericidin A1
Date:June 13 2025Web Page No:520084
Piericidin A1 is isolated from the fermentation broth of Streptomyces and known as a specific inhibitor of respiratory chain in electron transport system1).
The bioactive compound was reported as “piericidin A” initially, the detailed structural analysis revealed that the compound actually had 4 different analogues. From this report, the initially identified compound was named as piericidin A1.2)
It was reported that piericidin A1 inhibited Complex I (NADH:ubiquinone oxidoreductase) in the respiratory chain by targeting to the ubiquinone binding site.3, 4)
In addition, it is suggested that piericidin A1 prevents up-regulation of GRP78 and exhibits cytotoxicity in glucose-deprived HT29 cells (etoposide-resistant).5)
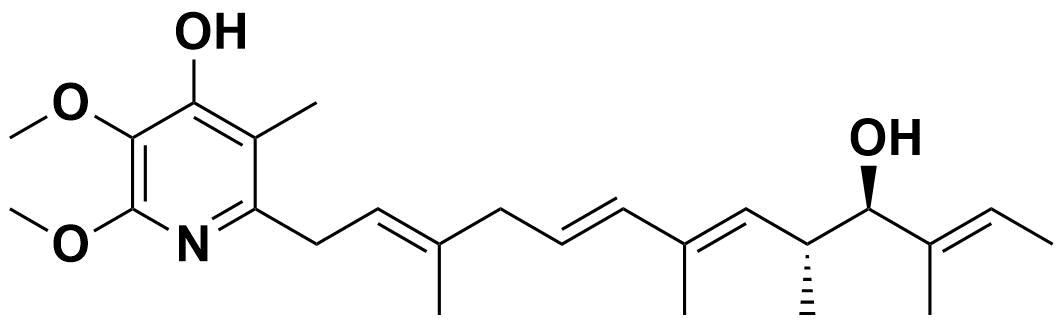
Structure of Piericidin A1 (#14688)
Specification
| CAS# | 2738-64-9 |
|---|---|
| Molecular Formula | C25H37NO4 |
| Molecular Weight | 415.574 |
| Purity | >90% (HPLC) |
| Supplied as | 1 mg/ml solution in EtOH* |
| Solubility | Soluble in MeOH, EtOH, hexane, DMSO, and DMF Hardly soluble in H2O |
* Piericidin A1 is an oil-like compound at room temperature. This product will be supplied as 3 vials of 1mL solution (1mg/mL) in EtOH. It is recommended a stock solution should be protected from light and use within a couple of days.
Application Note
- It was reported that piericidin A1 inhibited respiratory complex I (NADH:ubiquinone oxidoreductase) by targeting to the binding site of ubiquinone (IC50: 3.7 nM).3, 4)
- It was reported that piericidin A1 prevented up-regulation of GRP78 which acts as endoplasmic reticulum (ER) chaperon and exhibits cytotoxicity in glucose-deprived HT29 cells (etoposide-resistant).5)
Reference
- Tamura, S., et al., Agr. Biol. Chem., 27 (8), 576~582 (1963). [DOI:10.1271/bbb1961.27.576]
- Yoshida, S., et al., Agr. Biol. Chem., 41 (5), 849~853 (1977). [DOI:10.1271/bbb1961.41.849]
- Okun, J.G., et al., J. Biol. Chem., 274 (5), 2625~2630 (1999). [PMID:9915790]
- Zhou, X., et al., J. Antibiot., 69 (6), 582~593 (2016). [PMID:27301663]
- Hwang, J.H., et al., J. Cell. Physiol., 215 (1), 243~250 (2008). [PMID:17941090]

[Date : March 03 2026 00:09]
| Detail | Product Name | Product Code | Supplier | Size | Price | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Piericidin A1, NADH:Ubiquinone Oxidoreductase Inhibitor DatasheetThis may not be the latest data sheet. |
14688 | IMCInstitute of Microbial Chemistry | 3x1 mg | $500 | |||||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||||||||
[Date : March 03 2026 00:09]
Piericidin A1, NADH:Ubiquinone Oxidoreductase Inhibitor
DatasheetThis may not be the latest data sheet.
- Product Code: 14688
- Supplier: IMC
- Size: 3x1mg
- Price: $500
| Description |
M.W.: 415.574 Purity : >90% (HPLC) Molecular Formula : C25H37NO4 Supplied as: EtOH solution (1 mg/mL) Solubility : Soluble in MeOH, EtOH, hexane, and DMF, Hardly soluble in H2O. Piericidin A1 is a bioactive compound which is known as a specific inhibitor of the respiratory chain in the electron transport system. This compound inhibits functions of the respiratory chain by targeting to the ubiquinone binding site on the respiratory complex I (also known as NADH:ubiquinone oxidoreductase). |
||
|---|---|---|---|
| Storage | -20°C,Dark Storage | CAS | 2738-64-9 |
| Link | |||
CONTACT
export@funakoshi.co.jp
- ※Prices on our website are for your reference only. Please inquire your distributor for your prices.
- ※Please note that Product Information or Price may change without notice.