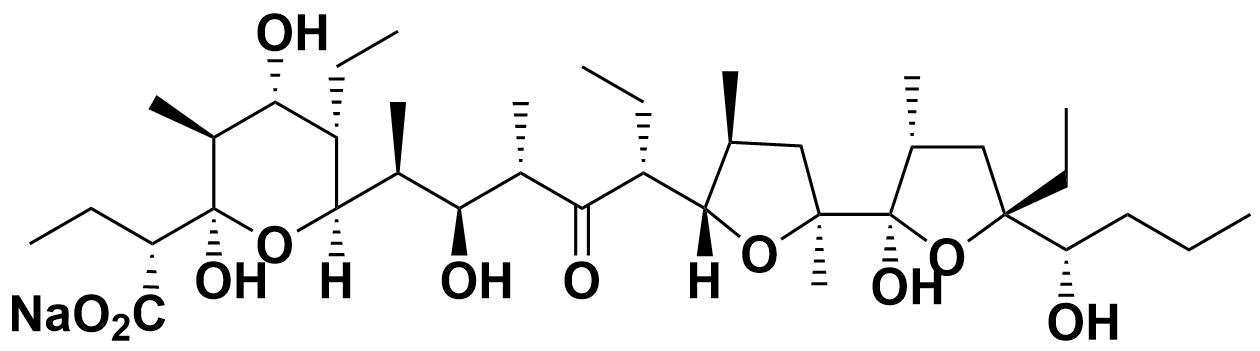
Inhibitor of Phosphatidylinositol Turnover Inostamycin A (sodium salt)
Date:July 03 2020Web Page No:520050
Inostamycin A is isolated from the culture fluid of Streptomyces sp. MH816-AF15 and inhibits cytidine diphosphodiacylglycerol (CDP-DG): inositol transferase 1).
Specification
| CAS# | 1884611-95-3 |
|---|---|
| Molecular Formula | C38H67O11 Na |
| Molecular Weight | 722.933 |
| Source | Streptomyces sp. MH816-AF15 |
| Purity | > 90% (Quantitative NMR) |
| Solubility | Soluble in DMSO, DMF and CHCl3. Poorly soluble in H2O |
Mechanism
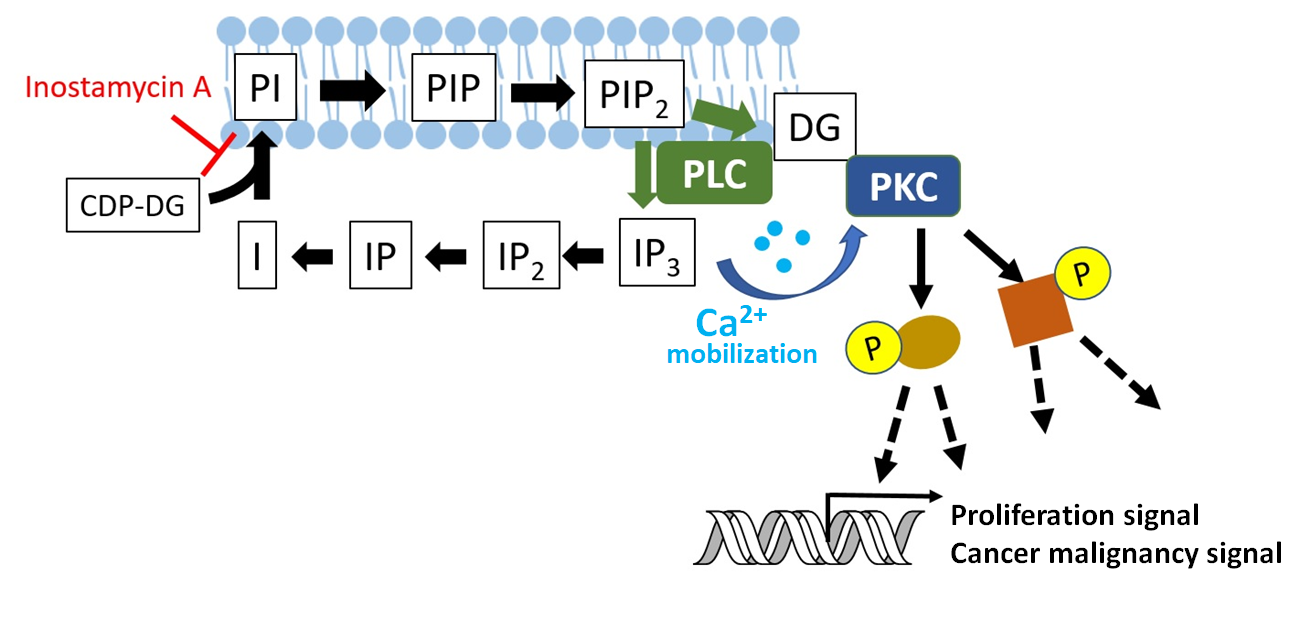
Phosphatidylinositol 2-phosphate (PIP2), generated from phosphatidylinositol (PI) present in the cell membrane, is degraded by phospholipase C (PLC) to inositol triphosphate (IP3) and diacylglycerol (DG), and IP3 is converted to inositol (I). The inositol (I) is bound to CDP-DG and PI is resynthesized. This metabolic turnover is called the inositol phospholipid turnover. IP3 generated by this turnover releases Ca2+ from the endoplasmic reticulum, and the Ca2+ and DG activate protein kinase C (PKC), which phosphorylates various intracellular proteins to activate proliferation signals and cancer malignancy signals.
Inostamycin A inhibits the synthesis of PI from I and CDP-DG in inositol-phospholipid-turnover and arrests cell-cycle in G1 phase, thereby inhibiting cell proliferation 2).
Application Note
- Inhibited in vitro CDP-DG: inositol transferase activity of the A431 cell membrane, the IC50 was about 0.02 μg/ml 1)
- Inhibited EGF-induced inositol incorporation into inositol lipids with an IC50 of about 0.5 μg/ml in the A431 cell assay system 2)
- Showed antimicrobial activities against Gram-positive bacteria and cytocidal activity against srk-NIH-3T3 cell with IC50 value of 0.07 μg/ml 3)
- Potenciated colchitine cytotoxicity toward KB-C4 cells at 3 μg/ml 4)
- Enhanced the cytotoxic effect of Taxol on small cell lung cancer cells 5)
- Caused Ms-1 cell to accumulate in the G1 phase at 0.1 μg/ml and induces morphological apoptosis at high concentration (0.3 μg/ml) 6)
Reference
- Imoto M. et al. J. Biochem., 112, 299~302 (1992)
- Imoto M. et al. J. Nat. Prod., 53(4), 825~829 (1990)
- Odai H. et al. J. Antibiot., 47(8), 939~941 (1994)
- Kawada M. et al. J. Antibiot., 45(4), 556~562 (1992)
- Imoto M. et al. Jpn. J. Cancer Res., 89(9), 315~322 (1998)
- Simizu S. et al. Jpn. J. Cancer Res., 89(9), 970~976 (1998)

[Date : February 15 2026 00:07]
| Detail | Product Name | Product Code | Supplier | Size | Price | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Inostamycin A (Sodium Salt), Antitumor DatasheetThis may not be the latest data sheet. |
14652 | IMCInstitute of Microbial Chemistry | 1 mg | $500 | |||||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||||||||
[Date : February 15 2026 00:07]
Inostamycin A (Sodium Salt), Antitumor
DatasheetThis may not be the latest data sheet.
- Product Code: 14652
- Supplier: IMC
- Size: 1mg
- Price: $500
| Description |
M.W.: 722.933 (Sodium salt) Purity : >90% (qNMR) Molecular Formula : C38H68O11Na (Sodium salt) Solubility : Soluble in DMSO, DMF, and CHCl3, Poorly soluble in H2O. Inostamycin A is an inhibitor of phosphatidylinositol turnover, supplied as sodium salt. It was reported that this compound inhibited EGF-induced inositol incorporation into inositol lipids in the A431 cell assay system. It was also reported that inhibition of in vitro CDP-DG: inositol transferase activity of the A431 cell membrane, antimicrobial activities against gram-positive bacteria, cytocidal activity against srk-NIH-3T3, and potentiated colchicine cytotoxicity toward KB-C4 cells. In addition, it caused Ms-1 cell to accumulate in the G1 phase and induced morphological apoptosis at high concentration. |
||
|---|---|---|---|
| Storage | -20°C | CAS | 1884611-95-3 (sodium salt), 129905-10-8 (salt free form) |
| Link | |||
CONTACT
export@funakoshi.co.jp
- ※Prices on our website are for your reference only. Please inquire your distributor for your prices.
- ※Please note that Product Information or Price may change without notice.