High Sensitive Denatured-Collagen Detection Reagent BindCOL, biotin-conjugated | Funakoshi
Date:November 19 2020Web Page No:95002

Funakoshi Co.,Ltd.
BindCOL, biotin-conjugated is a cyclic peptide reagent designed for the simple and highly sensitive detection of denatured collagen - an emerging disease marker. Thanks to its biotin label, detection is facilitated using various avidin or streptavidin-based systems. Unlike antibodies, this reagent specifically recognizes only denatured collagen, making it invaluable for research in collagen physiology and pathology.
It can also detect single-stranded collagen during the intracellular biosynthesis process. Please check ☞ here for more details.
- Background
- Mechanism
- Features
- Application
- Detection of single-chain collagen in biosynthetic process
- Product information
Background
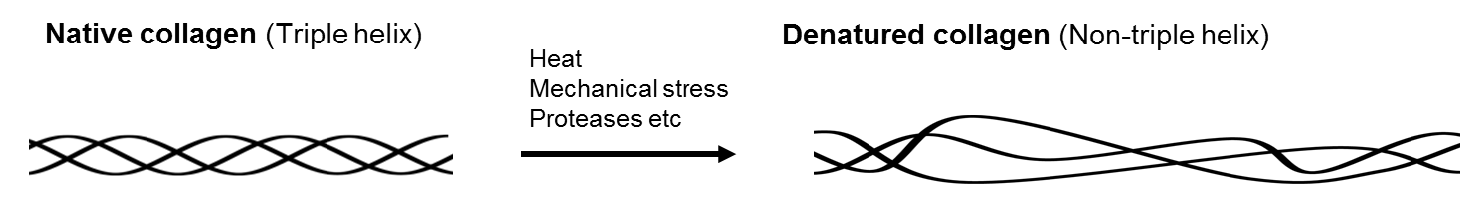
Collagen is a major component of the extracellular matrix and the most abundant protein in mammals. Its structure features repeated Gly‑Pro‑Hyp (4‑hydroxyproline) sequences, forming a triple-helical conformation. In humans, 27 collagen family members have been identified. Under proteolytic degradation or mechanical stress, the triple helix may unwind, producing denatured collagen (dCOL), which serves as a valuable biomarker in diverse pathological conditions, including tissue injury, osteoarthritis, fibrosis, inflammation, developmental processes, and aging.
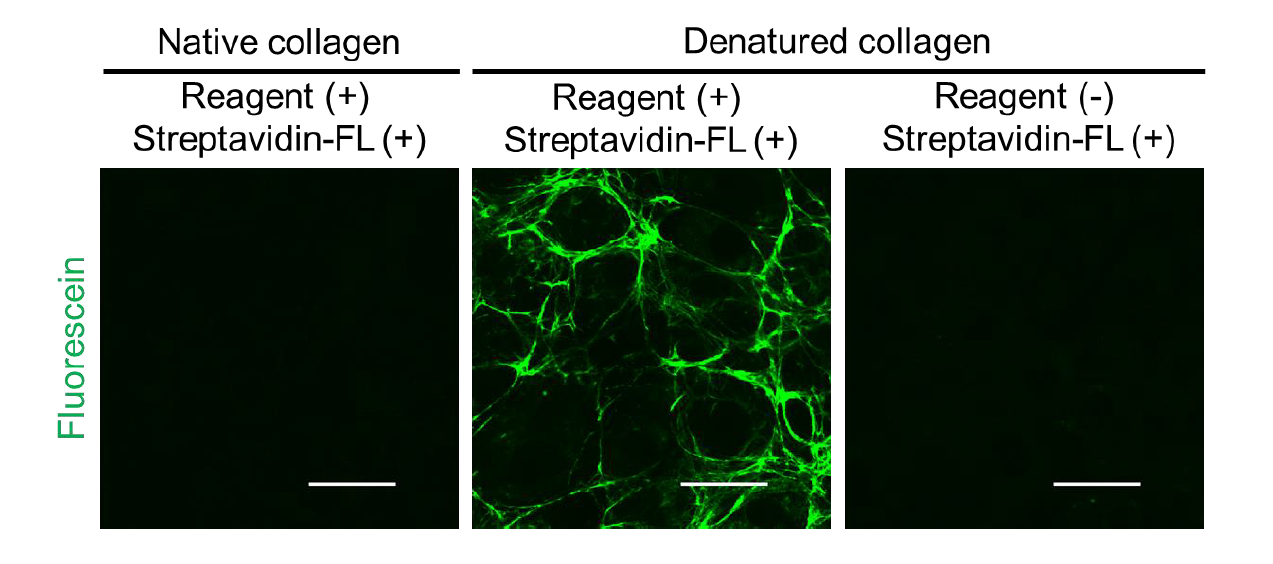
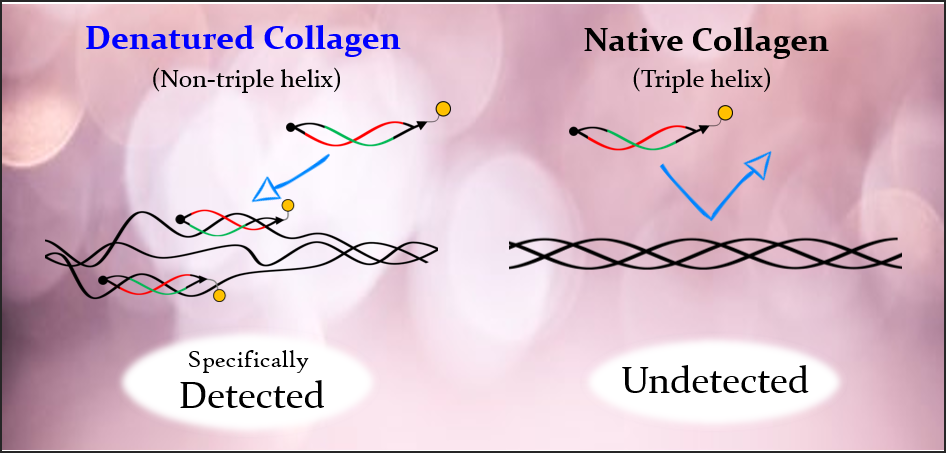
Although previous methods using collagen antibodies could not distinguish between native and denatured forms, BindCOL’s "strained cyclic peptide" technology enables highly sensitive and specific detection of denatured collagen.
Diagram of Denatured Collagen
Advantage
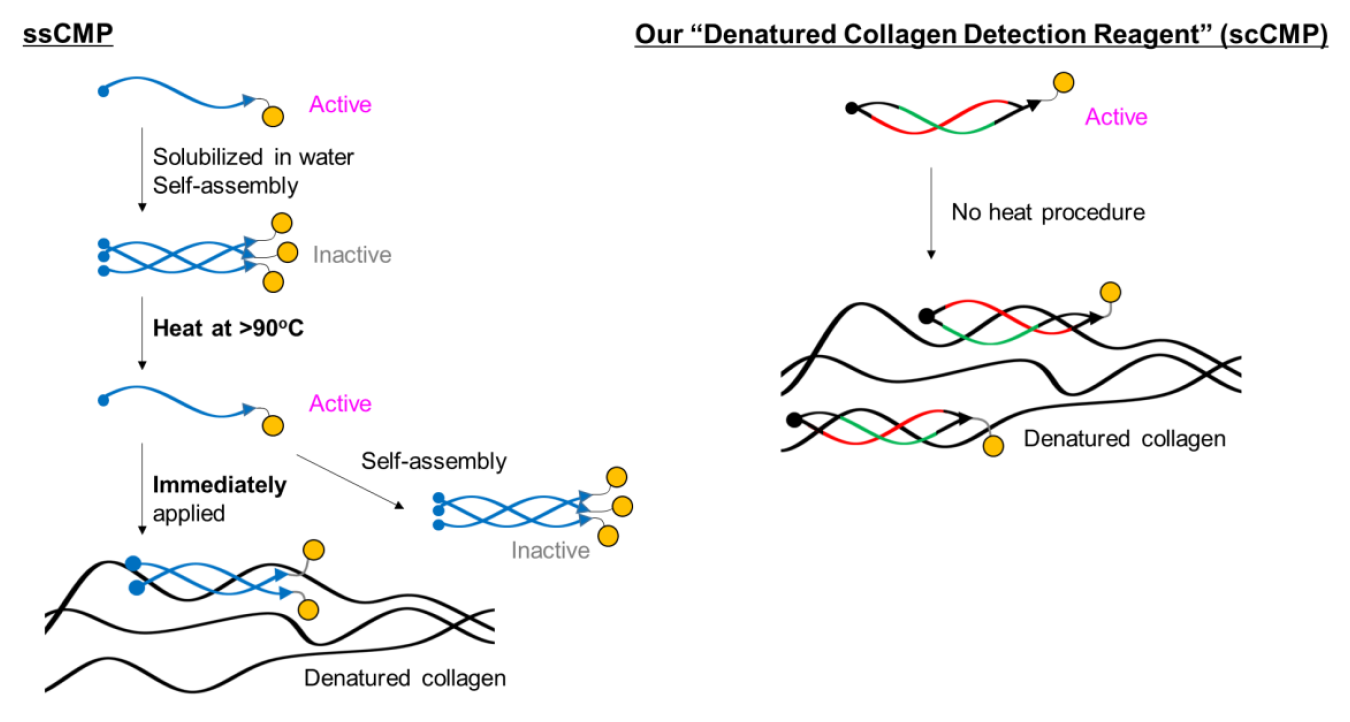
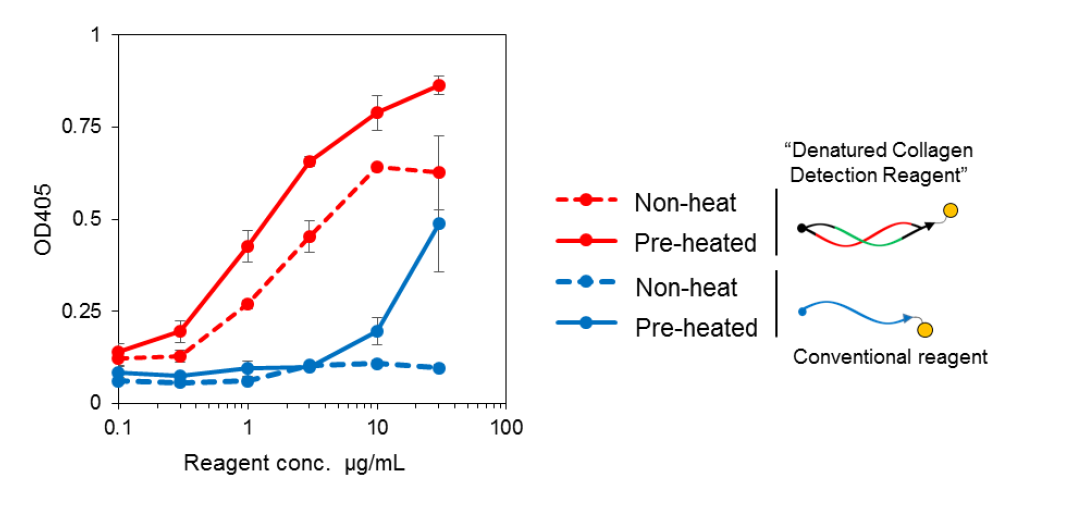
BindCOL stands out due to its use of strained cyclic peptide (scCMP) technology. Unlike conventional single-stranded collagen-mimetic peptides (ssCMPs) that tend to self-assemble into nonfunctional homo-trimers—necessitating a pre-heating step—BindCOL’s cyclic design, with varied backbone lengths, maintains a twisted structure that prevents self-association. This enhances binding affinity dramatically, enabling high-sensitivity detection without pre-heating.
Comparison of single-stranded and strained cyclic CMPs
Mechanism
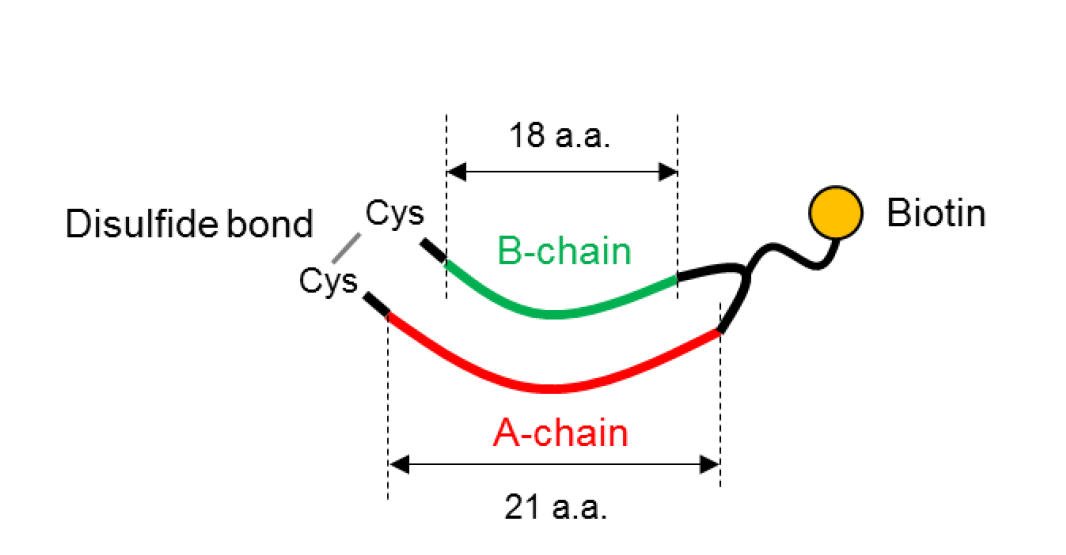
scCMP consists of two peptides with different length and biotin.
Reference
- Takita, K. K., Fujii, K. K., Ishii, K., Koide, T., Org. Biomol. Chem., 17, 7380~7387 (2019).
Structural optimization of cyclic peptides that efficiently detect denatured collagen. - Takita, K. K., Fujii, K. K. Kadonosono, T., Masuda, R., Koide, T., ChemBioChem., 19, 1613~1617 (2018).
Cyclic Peptides for Efficient Detection of Collagen.
Features
- Selective Binding: Targets only denatured collagen, not native triple helix
- High Sensitivity: Superior performance to ssCMP-based reagents, even without heating
- Versatile Detection: Compatible with avidin/streptavidin detection systems (fluorescent or HRP)
Comparison of binding affinity with conventional reagent
Application
Detection of denatured collagens derived from cultured cells
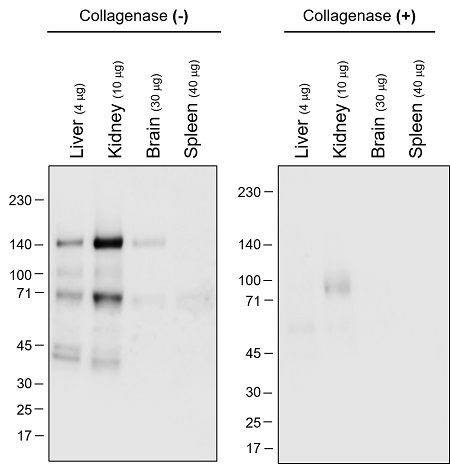
Detection of collagen members by Western Blotting
Detection of total collagen in tissue and validation of its specificity
Detection of single-chain collagen in biosynthetic process
Collagen biosynthesis takes place in the endoplasmic reticulum (ER), where it is synthesized as a single-stranded polypeptide, and then a triple helix is formed by collagen-specific chaperone HSP47 (procollagen). It is then secreted to the extracellular space via the Golgi apparatus, where it becomes mature collagen. It has been suggested that the accumulation of immature collagen in the endoplasmic reticulum (ER) due to deficiency/dysfunction of chaperone proteins or abnormal secretory pathway leads to disease. BindCOL can detect not only denatured collagen in the extracellular matrix, but also collagen in the biosynthetic process in the ER by using the same protocol as immunocytochemical staining. It is expected to be an excellent method for detecting single-chain collagen in the biosynthetic process, such as comparing the accumulation of abnormal collagen in normal and diseased cells.
Example of detection of single-chain collagen in cellular biosynthetic process
Product information
[Date : February 12 2026 00:09]
| Detail | Product Name | Product Code | Supplier | Size | Price | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
BindCOL, biotin-conjugated, Denatured Collagen Detection Reagent DatasheetThis may not be the latest data sheet. |
FDV-0035 | FNAFunakoshi Co.,Ltd. | 60 µg | $400 | |||||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||||||||
[Date : February 12 2026 00:09]
BindCOL, biotin-conjugated, Denatured Collagen Detection Reagent
DatasheetThis may not be the latest data sheet.
- Product Code: FDV-0035
- Supplier: FNA
- Size: 60µg
- Price: $400
| Description |
Denatured Collagen Detection Reagent (DCDR) is high sensitive and specific detection reagent for denatured collagen which is considered as novel pathological markers |
||
|---|---|---|---|
| Storage | -20°C | CAS | |
| Link |
|
||
CONTACT
export@funakoshi.co.jp
- ※Prices on our website are for your reference only. Please inquire your distributor for your prices.
- ※Please note that Product Information or Price may change without notice.