HOME>
Products>
Cell Culture>
Coating>
iMatrix-511 / iMatrix-511 silk
HOME>
Products>
Cell Culture>
Stem cells>
iMatrix-511 / iMatrix-511 silk
Suitable Substrate for Culturing Stem Cells (ES/iPS Cells) iMatrix-511 / iMatrix-511 silk
Date:August 13 2018Web Page No:46137

iMatrix-511 is a high-purity cell culture substrate purified from the E8 fragment of Laminin-511. It enables feeder-free culture and single-cell passaging of ES/iPS cells. iMatrix-511 Master Cell Bank Change Date: April 1, 2022 The iMatrix-511 (#892011 and #892012) master cell bank will be changed from May 2022.
The use of iMatrix-511 for human iPS cell culture is expected to contribute to the simplification of culture procedures *.
For more information, please click here.
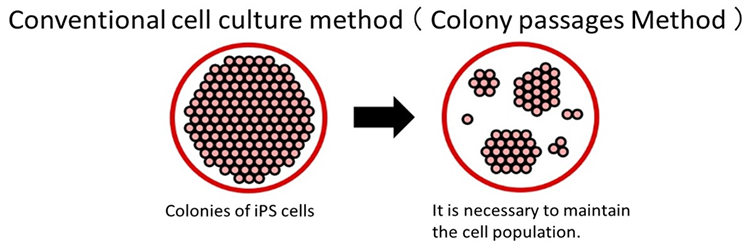
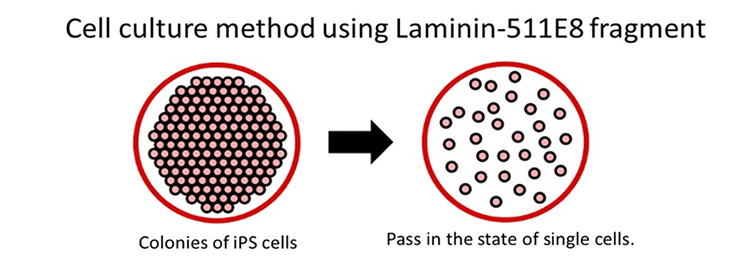
* Traditionally, the recommended method for using iMatrix-511 was to coat the container when culturing iPS cells. However, a group led by Associate Professor Hirofumi Suemori of the Institute for Virology and Regenerative Medicine at Kyoto University and Special Assistant Professor Takamichi Miyazaki of the Center for Integrated Material-Cell Systems developed a method for expanding human pluripotent stem cells that does not require coating.
- iMatrix Lineup
- Features
- Product Type
- How to Use
- Example of Use
- Advantages using Laminin-511 E8 Fragment
- What is Laminin-511 E8 fragment
- Citation
- Product Information
iMatrix Lineup
Features
- Feeder-free culture is possible.
- Single cell passaging is possible.
- Can be used for various cells - especially iPS / ES cells
- The addition of ROCK inhibitor is not required for medium change after passaging.
- We recommend coating with 0.5 μg/cm2 for iPS cell production and maintenance culture.
- Although iMatrix-511 silk is derived from a different organism than iMatrix-511, it has the same integrin-binding activity and no difference in usage or performance.
Product Type
| Name | iMatrix-511 | iMatrix-511 silk | |
|---|---|---|---|
| Product appearance |  |
 |
|
| Code | 892011 | 892012 | 892021 |
| Package | 2×175 μg | 6×175 μg | 6×175 μg |
| Expressed in/ Produced by |
Recombinant CHO-S cells | Recombinant silkworm | |
| Derived from | CHO-S cell culture supernatant | Silkworm cocoon | |
| Product grade | For research use | ||
| Transgene | Human Laminin-511 E8 fragment | ||
| Purity | 95% and more | ||
| Concentration | 0.5 mg/mL | ||
| Dissociation constant | 10 nM and under | ||
| Expiration date | 2 years after manufacture | ||
How to Use
- Dilute this product with PBS(-) and transfer to a cell culture device so that the concentration becomes 0.1-1.5 μg/cm2 *.
- Incubate at room temperature for 3 hours and discard the solution.
- Add the cells and culture medium and culture the cells.
* The optimum concentration depends on the type of cells.
■ Click here to view the expansion culture protocol for hES/hiPS cells using iMatrix-511.
■ Click here to view a video of hES/hiPS cell passaging using iMatrix-511.
Example of Use
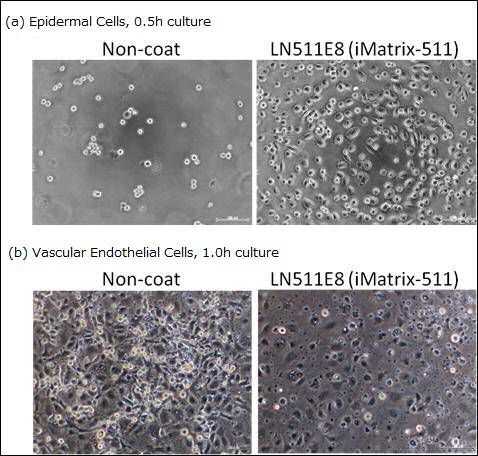
Evaluation of cell adhesion and spreading effect of Laminin-511 E8 using epidermal cells and vascular endothelial cells
Comparison of adhesion activity of full-length Laminin-511 and Laminin-511 E8 fragment in human ES cells
Comparison of cell growth in ES/iPS cell cultures using Laminin-511 E8 and Matrigel
Advantages using Laminin-511 E8 Fragment
What is Laminin-511 E8 fragment
Reference
- Miner, JH.,et. al., Annu. Rev. Cell Dev. Biol. 20, 255-284 (2004).
- Ido, H.,et. al., J. Biol. Chem. 279, 10946-10954 (2004).
- Ido, H.,et. al., J. Biol. Chem. 282, 11144-11154 (2007).
- Taniguchi, Y.,et. al., J. Biol. Chem. 284, 7820-7831 (2009).
- Miyazaki, T., et. al., Nat. Commun. 3, 1236 (2012).
Citation
| Classification | References | Topics |
|---|---|---|
| Establishment and Culture of Human Pluripotent Stem Cells (hPSCs) | Miyazaki, et al., Nat. Commun.3:1236, (2012) | Usefulness of hPSCs as a culture substrate |
| Nakagawa, et al., Sci. Rep. 4:3594, (2014) | Establishment of medical-grade hPSCs | |
| Takashima, et al., Cell.158(6):1254-69, (2014) | Contributes to the transition of hPSCs to the ground state | |
| Miyazaki, et al., Sci. Rep.7:41165, (2017) | Cultivation of hPSCs by Addition without Coating | |
| Sekine, et al., Stem Cell Res.24:40-43, (2017) | Establishment of disease-specific hPSCs | |
| Tan, et al., Stem Cell Res. 24:12-15, (2017) | ||
| Ishida, et al., Sci. Rep. 8(1), 310, (2018) | Generation of genetic disease models by gene editing of hPSCs | |
| Kim, et al., Nature Communications, 9(1), 939, (2018) | ||
| Sakai-Takemura, et al., Sci. Rep, 8, 6555, (2018) | Suspension culture of muscle progenitor cells differentiated from hPSCs | |
| Ling Li, et al., Experimental Neurobiology, 27(5), 350-364, (2018) | Establishment of disease-specific hPSCs from patients; Alzheimer's disease | |
| Cells induced to differentiate from hPSCs | Doi, et al., Stem Cell Reports. 2(3):337-50, (2014) | Dopamine-producing neurons |
| Ishikawa, et al., Hum. Mol. Genet.25(23):5188-5197, (2016) | ||
| Nishimura, et al., Stem Cell Reports.6(4):511-524, (2016) | ||
| Samata, et al., Nat. Commun. 7:13097, (2016) | ||
| Kikuchi, et al., Nature. 548(7669):592-596, (2017) | ||
| Morizane, et al., Nat. Commun.8(1):385, (2017) | ||
| Kikuchi, et al., J. Neurosci. Res.95(9):1829-37, (2017) | ||
| Goparaju, et al., Sci. Rep. 7:42367, (2017) | Motor neurons | |
| Burridge, et al., Nat. Methods.11(8):855-60, (2014) | Cardiomyocytes | |
| Sougawa, et al., Sci. Rep,8(1), 3726, (2018) | ||
| Yamauchi, et al., BBRC, 495(1), 1278-1284, (2018) | Ventricular-like cells | |
| Akiyama, et al., Sci. Rep, 8(1), 1189, (2018) | Skeletal muscle cells | |
| Saito et al. Stem Cell Res Ther, 9(1), 12, (2018) | Osteoblasts | |
| Uchimura, et al., Stem cell research, 25, 98-106, (2017) | Myoblasts | |
| Hayashi, et al., Nature.531(7594):376-80, (2016) | Photoreceptor cells | |
| Hayashi, et al., Nat. Protoc.12(4):683-696, (2017) | Corneal epithelial cells | |
| Takayama, et al., BBRC. 474(1):91-96, (2016) | Biliary epithelial cells | |
| Takayama, et al., Hepatol Commun, 1(10), 1058-1069, (2017) | Hepatocyte-like cells | |
| Takayama, et al., Biomaterials, (2018) | ||
| Takebe, et al., Cell Reports, 21(10), 2661-2670, (2017) | Hepatoblast | |
| Tan, et al., Stem Cell Reports, 11:1-11, (2018) | ||
| Camp, et al., Nature. 546(7659):533-38, (2017) | Definitive endoderm cells | |
| Zhang, et al., Stem Cell Reports, 10(2), 1?14, (2018) | Posterior endoderm progenitor cells | |
| Tanigawa, et al., Cell reports, 15(4), 801-813, (2016) | Nephron progenitor cells (fetal kidney cells) | |
| Musah, et al., Nat.Biomed.Eng.1:0069, (2017) | Glomerular epithelial cells | |
| Musah, et al., Nature protocols, 13(7):1662, (2018) | ||
| Mae, et al., BBRC, 495(1), 954-961, (2018) | Ureteric bud | |
| Oshima, et al., BBRC, 497(2), 719-725, (2018) | Common progenitor cells for blood cells and vascular endothelium | |
| Taguchi, et al., Cell Stem Cell, 21, (2017) | Culturing hPSCs to differentiate into nephron progenitor cells (fetal kidney cells) | |
| Kawamura, et al., Stem Cell Reports. 6(3):312-20, (2016) | Culturing hPSCs for cardiomyocyte differentiation | |
| Sasaki, et al., Cell Stem Cell.17(2):178-94, (2015) | Culture of hPSCs for differentiation into germ cells | |
| Kojima, et al., Cell Stem Cell.21(4):517-532, (2017) | ||
| Furuta, et al., PLoS One. 9(12):e112291, (2014) | Culturing hPSCs for differentiation into mesenchymal stem cells | |
| Primary human cell culture | Okumura, et al., Invest. Ophth. Vis. Sci.56(5):2933-42, (2015) | Human Corneal Endothelial Cells |
| Hongo, et al., Invest. Ophth. Vis. Sci. 58(9):3325-34, (2017) | ||
| Polisetti, et al., Sci. Rep.7(1):5152, (2017) | Human limbal epithelial progenitor cells | |
| Ishii, et al., Stem Cell Reports, 10, 1-15, (2018) | Satellite cell | |
| Molecular mechanisms of laminin-integrin interactions | Ido, et al., J. Biol. Chem. 282(15): 11144-54, (2007) | |
| Ido, et al., J. Biol. Chem.283(42): 28149-57, (2008) | ||
| Taniguchi, et al., J. Biol. Chem. 284(12): 7820-31, (2009) | ||
| Taniguchi, et al., BBRC.487(3): 525-531, (2017) | ||
| Takizawa, et al., Sci Adv.3(9) :e1701497, (2017) |
Product Information
[Date : February 25 2026 00:09]
| Detail | Product Name | Product Code | Supplier | Size | Price | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
iMatrix-511 (0.5 mg/mL solution) DatasheetThis may not be the latest data sheet. |
892011 | MAX | 2x175 µg | $290 | |||||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||||||||
|
iMatrix-511 (0.5 mg/mL solution) DatasheetThis may not be the latest data sheet. |
892012 | MAX | 6x175 µg | $787 | |||||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||||||||
|
iMatrix-511 silk DatasheetThis may not be the latest data sheet. |
892021 | MAX | 6x175 µg | - | |||||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||||||||
[Date : February 25 2026 00:09]
iMatrix-511 (0.5 mg/mL solution)
DatasheetThis may not be the latest data sheet.
- Product Code: 892011
- Supplier: MAX
- Size: 2x175µg
- Price: $290
| Description |
The recombinant human Laminin-511 E8 fragment, the coating material / substrate for iPS/ES cells culture |
||
|---|---|---|---|
| Storage | 4°C,Dark Storage | CAS | |
| Link |
|
||
iMatrix-511 (0.5 mg/mL solution)
DatasheetThis may not be the latest data sheet.
- Product Code: 892012
- Supplier: MAX
- Size: 6x175µg
- Price: $787
| Description |
The recombinant human Laminin-511 E8 fragment, the coating material / substrate for iPS/ES cells culture. |
||
|---|---|---|---|
| Storage | 4°C,Dark Storage | CAS | |
| Link |
|
||
iMatrix-511 silk
DatasheetThis may not be the latest data sheet.
- Product Code: 892021
- Supplier: MAX
- Size: 6x175µg
- Price: -
| Description |
iMatrix-511 silk is a recombinant human laminin-511 E8 fragment protein produced by a silkworm expression system. iMatrix-511 silk is a useful cell culture substrate for feeder-free culture and single-cell passage of ES cells and iPS cells, facilitating stable culture expansion. iMatrix-511 silk is also useful for the culture of other cells adhering to laminin-511. |
||
|---|---|---|---|
| Storage | 4°C,Dark Storage | CAS | |
| Link |
|
||
CONTACT
export@funakoshi.co.jp
- ※Prices on our website are for your reference only. Please inquire your distributor for your prices.
- ※Please note that Product Information or Price may change without notice.