抗HSP90 (Salmon)抗体 | Anti-HSP90 (Salmon) antibody
掲載日情報:2018/10/03 現在Webページ番号:26550
StressMarq Biosciences社の抗HSP90 (Salmon)抗体(Anti-HSP90 (Salmon) antibody)です。
※本製品は研究用です。研究用以外には使用できません。
カートに商品を
追加しました。
追加しました。
価格
[在庫・価格 :2026年02月22日 00時00分現在]
※ 表示されている納期は弊社に在庫が無く、取り寄せた場合の納期目安となります。
| 詳細 | 商品名 |
|
文献数 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Anti-Hsp90, Rabbit-Poly |
|
本製品は取扱中止になりました | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
[在庫・価格 :2026年02月22日 00時00分現在]
※ 表示されている納期は弊社に在庫が無く、取り寄せた場合の納期目安となります。
Anti-Hsp90, Rabbit-Poly
文献数: 2
- 商品コード:SPC-316D
- メーカー:STQ
- 包装:100μl
- 本製品は取扱中止になりました
| 説明文 | Genbank No:
100136360
Gene Accession No: NP_001117004.1 Protein Accession No: Q9W6K6 |
||
|---|---|---|---|
| 法規制等 | |||
| 保存条件 | 法規備考 | ||
| 抗原種 | Salmon | 免疫動物 | Rabbit |
| 交差性 | Fish/Human/Salmon | 適用 | Western Blot |
| 標識 | Unlabeled | 性状 | Serum |
| 吸収処理 | クラス | ||
| クロナリティ | Polyclonal | フォーマット | |
| 掲載カタログ |
|
||
| 製品記事 | Hsp90 Antibody And Protein Pack 熱ショックタンパク質研究用製品特集 |
||
| 関連記事 | |||
カートに商品を
追加しました。
追加しました。
製品情報
Product Name
HSP90 (Salmon) Antibody
Clonality
Polyclonal
Description
Rabbit Anti-Salmon HSP90 (Salmon) Polyclonal
Research Areas
Cancer, Heat Shock, Cell Signaling, Chaperone Proteins, Protein Trafficking, Tumor Biomarkers
Alternative Names
HSP84 Antibody, HSP86 Antibody, HSP90A Antibody, HSP90AA1 Antibody, HSP90AB1 Antibody, HSP90B Antibody, HSPC1 Antibody, HSPC2 Antibody, HSPCAL1 Antibody, HSPCAL4 Antibody, HSP90N Antibody
Host Species
Rabbit
Immunogen
KLH-conjugated synthetic peptide chosen from a highly conserved region of HSP90 found in both the alpha and beta form. The taget peptide is perfectly conserved in animals.
Applications
WB
Species Reactivity
Human, Fish, Salmon
Accession Number
NP_001117004.1
Gene ID
100136360
Swiss Prot
Q9W6K6
Specificity
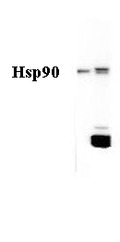
Detects ~90kDa. In salmonid fish a cross-reactive band at 40kDa is observed. Antibody will also detect a Human recombinant HSP90 protein.
Purification
Rabbit antiserum
Storage Buffer
Lyophilized rabbit Antiserum. For reconstitution add 100 µl of sterile water.
Certificate of Analysis
0.2 µl/ml of SPC-316 was sufficient for detection of HSP90 in 10 µg Heat shocked (25°C) rainbow trout nuclear fraction lysate by colorimetric immunoblot analysis using Goat anti-rabbit IgG:HRP as the secondary antibody.
References
Scientific Background
HSP90 is an abundantly and ubiquitously expressed heat shock protein. It is understood to exist in two principal forms α and β, which share 85% sequence amino acid homology. The two isoforms of HSP90, are expressed in the cytosolic compartment (1). Despite the similarities, HSP90α exists predominantly as a homodimer while HSP90β exists mainly as a monomer.(2) From a functional perspective, HSP90 participates in the folding, assembly, maturation, and stabilization of specific proteins as an integral component of a chaperone complex. (3-6) Furthermore, HSP90 is highly conserved between species; having 60% and 78% amino acid similarity between mammalian and the corresponding yeast and Drosophila proteins, respectively. HSP90 is a highly conserved and essential stress protein that is expressed in all eukaryotic cells. Despite it’s label of being a heat-shock protein, HSP90 is one of the most highly expressed proteins in unstressed cells (1–2% of cytosolic protein). It carries out a number of housekeeping functions – including controlling the activity, turnover, and trafficking of a variety of proteins. Most of the HSP90-regulated proteins that have been discovered to date are involved in cell signaling. (7-8). The number of proteins now know to interact with HSP90 is about 100. Target proteins include the kinases v-Src, Wee1, and c-Raf, transcriptional regulators such as p53 and steroid receptors, and the polymerases of the hepatitis B virus and telomerase.5 When bound to ATP, HSP90 interacts with co-chaperones Cdc37, p23, and an assortment of immunophilin-like proteins, forming a complex that stabilizes and protects target proteins from proteasomal degradation. In most cases, HSP90-interacting proteins have been shown to co-precipitate with HSP90 when carrying out immunoadsorption studies, and to exist in cytosolic heterocomplexes with it. In a number of cases, variations in HSP90 expression or HSP90 mutation has been shown to degrade signaling function via the protein or to impair a specific function of the protein (such as steroid binding, kinase activity) in vivo. Ansamycin antibiotics, such as geldanamycin and radicicol, inhibit HSP90 function (9). Looking for more information on HSP90? Visit our new HSP90 Scientific Resource Guide.
References
1. Nemoto T., et al. (1997) J.Biol Chem. 272: 26179-26187.
2. Minami Y., et al. (1991), J.Biol Chem. 266: 10099-10103.
3. Arlander S.J.H., et al. (2003) J Biol Chem 278: 52572-52577.
4. Pearl H., et al. (2001) Adv Protein Chem 59:157-186.
5. Neckers L., et al. (2002) Trends Mol Med 8:S55-S61.
6. Pratt W., Toft D. (2003) Exp Biol Med 228:111-133.
7. Pratt W., Toft D. (1997) Endocr Rev 18:306–360.
8. Pratt W.B. (1998) Proc Soc Exptl Biol Med 217: 420–434.
9. Whitesell L., et al. (1994) Proc Natl Acad Sci USA 91: 8324–8328.
10. Barent R. L. (1998) Mol. Endocrinol. 12: 342-354
11. Lo. M.A. (1998) EMBO J. 17: 6879-6887.
HSP90 is an abundantly and ubiquitously expressed heat shock protein. It is understood to exist in two principal forms α and β, which share 85% sequence amino acid homology. The two isoforms of HSP90, are expressed in the cytosolic compartment (1). Despite the similarities, HSP90α exists predominantly as a homodimer while HSP90β exists mainly as a monomer.(2) From a functional perspective, HSP90 participates in the folding, assembly, maturation, and stabilization of specific proteins as an integral component of a chaperone complex. (3-6) Furthermore, HSP90 is highly conserved between species; having 60% and 78% amino acid similarity between mammalian and the corresponding yeast and Drosophila proteins, respectively. HSP90 is a highly conserved and essential stress protein that is expressed in all eukaryotic cells. Despite it’s label of being a heat-shock protein, HSP90 is one of the most highly expressed proteins in unstressed cells (1–2% of cytosolic protein). It carries out a number of housekeeping functions – including controlling the activity, turnover, and trafficking of a variety of proteins. Most of the HSP90-regulated proteins that have been discovered to date are involved in cell signaling. (7-8). The number of proteins now know to interact with HSP90 is about 100. Target proteins include the kinases v-Src, Wee1, and c-Raf, transcriptional regulators such as p53 and steroid receptors, and the polymerases of the hepatitis B virus and telomerase.5 When bound to ATP, HSP90 interacts with co-chaperones Cdc37, p23, and an assortment of immunophilin-like proteins, forming a complex that stabilizes and protects target proteins from proteasomal degradation. In most cases, HSP90-interacting proteins have been shown to co-precipitate with HSP90 when carrying out immunoadsorption studies, and to exist in cytosolic heterocomplexes with it. In a number of cases, variations in HSP90 expression or HSP90 mutation has been shown to degrade signaling function via the protein or to impair a specific function of the protein (such as steroid binding, kinase activity) in vivo. Ansamycin antibiotics, such as geldanamycin and radicicol, inhibit HSP90 function (9). Looking for more information on HSP90? Visit our new HSP90 Scientific Resource Guide.
References
1. Nemoto T., et al. (1997) J.Biol Chem. 272: 26179-26187.
2. Minami Y., et al. (1991), J.Biol Chem. 266: 10099-10103.
3. Arlander S.J.H., et al. (2003) J Biol Chem 278: 52572-52577.
4. Pearl H., et al. (2001) Adv Protein Chem 59:157-186.
5. Neckers L., et al. (2002) Trends Mol Med 8:S55-S61.
6. Pratt W., Toft D. (2003) Exp Biol Med 228:111-133.
7. Pratt W., Toft D. (1997) Endocr Rev 18:306–360.
8. Pratt W.B. (1998) Proc Soc Exptl Biol Med 217: 420–434.
9. Whitesell L., et al. (1994) Proc Natl Acad Sci USA 91: 8324–8328.
10. Barent R. L. (1998) Mol. Endocrinol. 12: 342-354
11. Lo. M.A. (1998) EMBO J. 17: 6879-6887.
カートに商品を
追加しました。
追加しました。
製品情報は掲載時点のものですが、価格表内の価格については随時最新のものに更新されます。お問い合わせいただくタイミングにより製品情報・価格などは変更されている場合があります。
表示価格に、消費税等は含まれていません。一部価格が予告なく変更される場合がありますので、あらかじめご了承下さい。